|
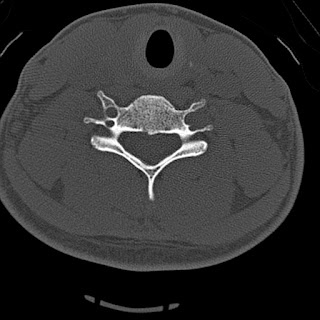
The Castellvi system for organizing lumbosacral variations.
Useful only for classification, not prognostic significance. |
The design of the spine is awkard enough as it is, with the majority of the body's weight being transferred through L5-S1, but as if to make an awkward system even a little more awkward, every so often congenital vertebral variations occur at the lumbosacral junction and confuses the typical layout (see
Hox genes in the 9/29/2011 post).
There are two main possibilities for a lumbosacral transitional vertebrae:
- "
lumbarization of S1"
- "
sacralization of L5"
which seem to be two ways of looking at the same thing... except in the first situation you essentially end up with an extra lumbar vertebra ("L6"), and in the second, you end up with one too few lumbar vertebrae (L1-L4). Note that, unlike the "gorilla bone" example from 9/29/2011, it's assumed that there are the normal 12 ribs in both these situations, but then again it's more common to have thoracolumbar variations if there is a lumbosacral variation...
... so the final result of all this madness is that the only absolutely sure way to know which variations you're dealing with in the spine is to count down from C2...
...and numbering of these segments is critical since they frequently get intervened upon, either by surgery or by anesthesia.
A recent study demonstrated that the iliolumbar ligament (described as "absent in 70%" in earlier studies), actually denotes the lowest lumbar vertebra,
which is not always L5 and should not be used as a marker for L5.
Some object to the use of "L6" as the best term for this lowest lumbarized S1 vertebra since there is no L6 nerve root. If "L6" is used, then the S1 nerve root would pass out beneath it, and then the S2 nerve root would passes out below/through the S1 neuroforamina...... and the whole vertebra-nerve root thing goes cattywhompus.
It is claimed by some, however, that the L5 nerve root always passes out the "last mobile" segment of the spine, so in a patient with a lumbarized S1 like the one below, the last mobile level is L6-S2, and the functional L5 nerve root corresponds to the "L6" nerve root. The point hold true for
patients with a sacralized L5 as well, and the L4 nerve root serves the usual function of the L5 nerve root. This point has been debated, and some feel that this nerve root also shares some aspects of S1... but as of today, no thorough study has determined which is correct (perhaps both).
One other side note: whether lumbosacral transitional vertebrae is related to low back pain is controversial. A recent study demonstrated that nearly a 1/3 of a normal population sample had at least minor changes of transitional lumbosacral vertebrae in the spine, implying that it may not be as closely associated with low back pain as was once thought. Another recent study positively correlated Castellvi types II and IV with an increase in low back pain.
---
1. Apazidis A, Ricart P, et al. "The Prevalence of Transitional Vertebrae in the Lumbar Spine."
The Spine Journal 11 (2011) 858-862
2. Kim YH, Lee PB, et al. "Dermatome Variation of Lumbosacral Nerve Roots in Patients with Transitional Lumbosacral Vertebrae"
Anesthesia & Analgesia 2008; 106:1279-83
3. Carrino JA, Campbell PD, et al. "Effect of Spinal Segment Variants on Numbering Vertebral Levels at Lumbar MR Imaging"
Radiology, 259, 196-202 April 2011
4. Nardo L, Alizai H, Virayavanich W. "Lumbosacral Transitional Vertebrae: Association with Low Back Pain" November 2012 Radiology,265, 497-503.